The Tufts CSDD Insider is the best way to stay connected with the Tufts Center for the Study of Drug Development. Published monthly to provide updates on center activity, The Insider details upcoming research studies, professional development courses, publications and presentations. The most recent issue of the Insider is available below. Sign up for our Monthly Insider


From the Executive Director
Dear CSDD Friends:
I’ve been thinking about Phil Needleman, who passed away last month. A pharmacology professor and department chair at Washington University’s School of Medicine, he also served as a senior R&D executive at Searle and then Pharmacia during the 1990s and early 2000s. Phil was very outspoken about the challenges and dysfunction in drug development. For me, one of his most illuminating truisms— or ‘commandments’ as he called them — is that in drug development “the world belongs to finishers.” The very best strategies and planning only succeed through strong execution. At a conference many years ago, I vividly recall Phil emphasizing the importance of personnel talent and professionalism, collaborative effectiveness, integrity, discipline, and the unyielding desire for the full team to see things through to the finish.
Ongoing commitment to optimize clinical research execution can be found in a number of Tufts CSDD projects underway and soon to begin. Two working group studies are fielding survey data this month: one profiling AI-enabled use cases supporting drug development planning and operations; another assessing inefficiencies in the vendor qualification and collaboration process.
Tufts CSDD has initiated a study validating a new tool that forecasts investigative site burden associated with protocol execution. And in April we’re launching three new studies — one assessing study volunteer and investigative site perceptions and experience with direct-to-patient investigational drug delivery; a second study looking at clinical data management practices and optimization opportunities; and a third examining the relationship between pre-approval clinical trial practices and new medical therapy adoption post-approval. Please contact me if you’re interested in learning about any of our ongoing or upcoming studies.
Please periodically check our monthly Insider and our website. They are great ways to monitor Tufts CSDD news, projects, workshops, course offerings, publications and presentations. And as always, we welcome your feedback, suggestions, inquiries and ideas.
All the best,

Kenneth Getz
Executive Director, Tufts CSDD
Professional Development Courses

Join our interactive online session tailored for emerging leaders in the pharma & biotech sectors moderated by Dr. Robert Franco. Ideal for those stepping into a supervisory role, this workshop offers valuable insights and strategies for success in your leadership position. This course will be held via Zoom on June 11, 13, 20, & 21st, 2024 from 11-3 pm EST. Course information coming soon! Sign up to be the first to know more at: https://csdd.tufts.edu/leadership or email Course Facilitator Sarah Wrobel.
News of Note

Last month, Tufts CSDD’s PALADIN Consortium launched a Playbook and a Repository of Resources for patient advocacy groups (PAG) and biopharmaceutical companies to use in optimizing their collaborations. The Playbook provides process maps and forms to initiate and guide collaborations as well as template service and confidentiality agreements to expedite PAG-industry engagements. The Resource Repository contains over 100 identified and assessed best-in-class operational resources with links to the organizations that developed them. Both the Playbook and the Resource Repository are accessible on Paladinconsortium.org and they are available free of charge.
Upcoming Studies

Investigative Site Participation Burden Tool Validation
Tufts CSDD has launched a new study to validate a tool designed to assess investigative site participation burden in clinical trials. Sponsors and CROs will apply the tool following a brief training exercise. Several companies have already joined the study. Please contact Maria Florez if your organization would like to learn more.
Research Highlights

Introducing Our March/April Impact Report
Workplace Microaggressions Associated with Staff Turnover and Poor Performance
Our March/April Impact Report (Volume 26, Number 2) presents the results of a groundbreaking new study quantifying the incidence and impact of gender and racial microaggressions among drug development professionals. The report also documents the association between microaggressions and employee turnover and poor clinical team performance.
Recent Publications
Lamberti M, Dirks A, Nicholas K, Cervantes N, Getz K. An Examination of the Use of Patient Recruitment and Retention Tactics for Global Studies. Applied Clinical Trials. March 18, 2024. Access article.
Botto E, Ford MR, Do H, Getz K. Patient and Site Personnel Perceptions of Retail Pharmacy Involvement in Clinical Research. Applied Clinical Trials. March 7, 2024. Access article.
Botto E, Smith Z, Getz K. New Benchmarks of Protocol Amendment Experience in Oncology Clinical Trials. TIRS. March 2024. Access article.
Getz K. Calling Out Check-the-Box Patient Engagement. Applied Clinical Trials. March 2024.
Nguyen DV, Block CJ, Kim JY, Yu H. General and Stereotype-Based Microaggresions Experienced by Asians and Asian Americans in the Workplace: A Qualitative Study. American Behavioral Scientist. March 1, 2024. Access article.
Getz K, Smith Z, Botto E, Murphy E, Dauchy A. New Benchmarks on Protocol Amendment Practices, Trends and their Impact on Clinical Trial Performance. TIRS. 2024. Access article.
Kim J, Florez M, Botto E, Bhagat R, Boynton K, Ferguson. Bridging the Recruitment Gap for Socioeconomically Disadvantaged Groups in Clinical Trials. Applied Clinical Trials. February 16, 2024. Access article.
Dirks A, Florez M, Torche F, Young S, Slizgi B, Getz K. Comprehensive Assessment of Risk-Based Quality Management Adoption in Clinical Trials. TIRS. February 2024. Access article.
Florez M, Smith Z, Olah Z, Martin M, Getz K. Quantifying Site Burden to Optimize Protocol Performance. TIRS. 2024. Access article.
Getz K. Shining a Light on Inefficiencies in Protocol Amendment Implementation. Applied Clinical Trials. Published December 6, 2023. Access article.
Smith Z, Getz K. A Case Study Assessment on the Rationale for, and Relevance of, Non-Core Protocol Data. Ther Innov Regul Sci. Published December 1, 2023. Access article.
Data Insights Digest
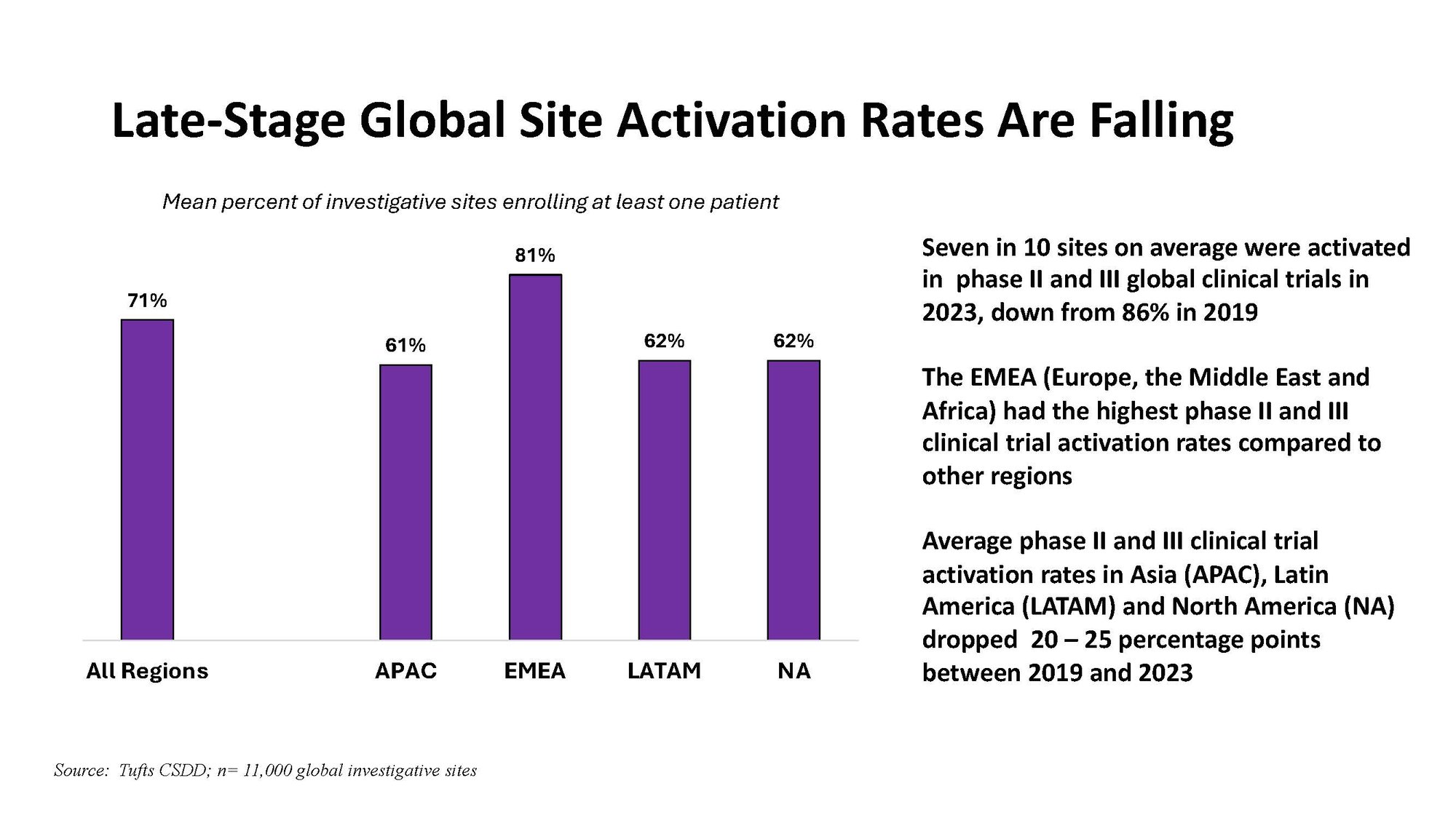
To access hard-hitting Tufts CSDD charts and tables, visit https://csdd.tufts.edu/impact-reports.
Faculty & Staff Presentations
Upcoming Presentations
DEI from Within: The Impact of Diverse Clinical Research Teams on Patient Inclusion
SCRS Site Solutions Summit
Atlanta, GA | April 8
DEI from Within: Keynote
SCRS Site Solutions Summit
Atlanta, GA | April 8
Navigating Academia. Colloquium.
Teachers College, Columbia University
New York, NY | April 9
Anticipating New Directions in Response to a Changing Drug Development Landscape
EQuaTR – NorthWestern University Feinberg School of Medicine
Chicago IL | April 10
Where has the Industry Been and Where Should it be Going?: Using Industry Development Benchmarks (Time, Risk, and Cost Metrics) to Improve Performance
Drug Development Boot Camp, Speid & Associates, Inc & Brown University Alpert Medical School
Virtual | April 11
Frequency and Impact of Protocol Amendments on Clinical Trial Performance
Festival of Biologics
San Diego, CA | April 15
Panel Discussion on Innovation Adoption
InformaConnect
Philadelphia, PA | April 16
Amplifying the Patient Voice in Drug Development
Reuter’s Pharma 2024
Barcelona | April 16
Quantifying the Financial Value of Digital Endpoints in Clinical Trials | Data Driven, Hybrid & Full Decentralized Trials
Informa Connect
Philadelphia, PA | April 16-17
Protocol Simplification or Optimization?
CMO Summit
Boston MA | April 17
Benchmarking Patient Enrollment and Use of Patient Recruitment Tactics in Global Trials
World BI
Boston, MA | May 2
Retail Pharmacies and Clinical Trials: Perspectives from Industry, Sites & Patients
Association of Clinical Research Professionals 2024
Anaheim, CA | May 4
Recent Presentations
Site Activation and Patients Enrollment Benchmarks Among Sponsors and CROs
Summit for Clinical Operations Executives (SCOPE)
Orlando, FL| February 14
Assessing Current Levels and Identifying Barriers to RBQM Adoption
Summit for Clinical Operations Executives (SCOPE)
Orlando, FL | February 14
Protocol Design Trends and Their Impact on Performance
Summit for Clinical Operations Executives (SCOPE)
Orlando, FL | February 14
Optimization in the Age of Hyper-Customization
Harvard – MIT Center for Regulatory Science
Virtual | March 5
Interactive Workshop: Upskilling for Successful Digital Transformations in Regulatory Affairs and Clinical Operations
DIA Europe
Brussels, BE | March 13
Amplifying and Applying Patient Voices in Protocol Planning and Design
Patients as Partners
Philadelphia, PA | March 22
Optimizing Study Design and Setting the Stage for Efficient Study Conduct Through Quality by Design
Robert J. Margolis Center for Health Policy at Duke University and the Food and Drug Administration
Washington DC | January 31
Measuring RBQM Adoption: Insights & Opportunities
Dynamic Global Events RBQM Summit
Philadelphia, PA | January 25
The Promise and Perils of Decentralized Clinical Trials (DCT)
Tufts CSDD – Cambridge University Joint Webinar
Virtual | January 22
Advancing Diversity in Clinical Trials: Insights from Recent Research
LinkedIn Live
Virtual | January 18

