
About this Course
This highly interactive online course, offered twice each year, brings together team leaders, program managers, functional directors, and other drug development professionals from across the industry to build leadership skills, improve cross-functional performance, and enhance R&D productivity. Delegates meet online in large and small groups over the course of four weeks.
Tufts CSDD holds the Leadership for Drug Development Teams course several times each year. Custom programs are also available for professionals within a single organization.
Objectives
Successfully lead multi-functional and multi-organizational teams, including joint ventures and academic partnerships Implement effective communication frameworks to create innovative high-performing teams
Effectively prepare for, conduct, and achieve positive senior management review meetings
Optimize outsourcing and vendor relationships to improve efficiency and maximize performance
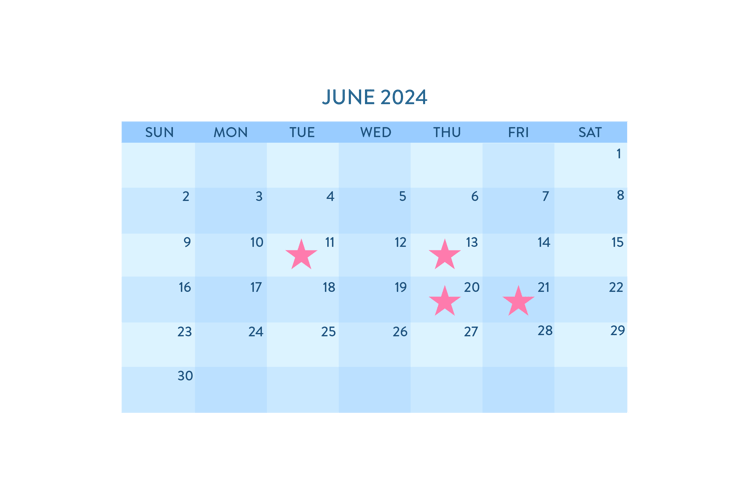
Summer 2024 Course Dates
June 11, 13, 20 & 21st, 2024 | Live Online | 11 - 3 pm EST
Summer 2024 Registration
Information Coming Soon
Group/Custom Rate

Earn a Digital Badge & Certificate!
- Showcase the knowledge and skills you acquired from the program.
- Share your learning achievements with clients, employers, and colleagues on online platforms and social media networks like LinkedIn, Facebook and Twitter.
“I was able to immediately make improvements after taking this course…Once others started seeing the way I interacted, I noticed that they mirrored the behavior. It’s made a difference in the team.” Program Manager, Sanofi
“I really appreciate that it was drug-development focused. The examples were so close to our real lives that it made them easy to implement. The course was perfectly suited to my professional development goals: to develop the skills I need for integrated work in a matrix organization.” Clinical Research Physician, AstraZeneca
“I enjoyed the workshop tremendously. The topics were relevant and coherent and the group interactions gave me many different perspectives. I am eager to practice the skills in real life.” Principal Scientist, Immuno
Course Moderator
Robert Franco, PhD
With over 24 years of consulting experience, Dr. Franco led PwC’s Pharmaceutical R&D practice where he specialized in improving pharmaceutical drug development, technology transfer, clinical trial operations, and manufacturing. Dr. Franco has worked with the senior management of several large pharmaceutical and biotechnology companies, NGOs, and multinational organizations to implement complex change management initiatives to improve growth, reduce costs, and remediate quality and regulatory issues. Dr. Franco has eight management consulting articles, 13 technical publications, and three patents to his name and has served on several international technical review and regulatory standards committees. He has a Doctor of Philosophy and Master of Science in biochemistry from the University of Rochester.
Course Facilitator
Sarah Wrobel, MHA
GET MORE INFORMATION
Have any questions? Enter your information below to get in touch with our enrollment team and access the course agenda.
