As an independent, globally-focused multidisciplinary academic team of researchers, Tufts CSDD conducts a variety of robust, data-driven assessments and analyses to derive new insights into strategies and practices that optimize drug development performance and efficiency. Each year, the Center’s research agenda focuses on priority and timely topics identified by Tufts CSDD faculty and based on suggestions by individual or multiple organizations. Research studies may entail fielding a survey; conducting interviews and in-depth individual and group discussions; gathering primary and secondary data; and performing sophisticated yet practical and highly relevant data analyses. To support our mission as an academic research center, the results of all Tufts CSDD studies are published in peer-review and trade journals. Tufts CSDD only publishes aggregate analyses — individual companies, specific drugs and biologics, and proprietary information and experiences are never identified. Participating organizations may also receive customized reports comparing their own performance, practice, and experience against aggregate industry benchmarks.About our Working Group Studies: Tufts CSDD is in a unique position to convene a group of companies looking to share experiences and data and to collectively review and discuss study findings and insights. These multi-company working group studies have generated compelling analyses, invaluable benchmarks, and novel insights into the drug development process as well as practices and solutions to drive performance improvement and efficiency. Examples of recently completed and active individually- and working group-funded studies:
-
NME drug development metrics by therapeutic class and molecule type
-
Trends in drug and biologic approvals -
Regulatory review cycle times and the impact of regulatory reform on performance -
Preclinical cycle times and economics -
Protocol design complexity and impact on site and patient participation burden -
Study conduct cycle times, patient recruitment and retention effectiveness -
Adoption and impact of patient engagement initiatives
-
Remote-based clinical teams: performance and best practices
-
Participant diversity, inclusion, and disparities in clinical trials -
Adoption and use of hybrid and virtual/decentralized models supporting clinical trials -
The use and impact of machine learning and artificial intelligence (AI) in clinical research -
The use of genetic and biomarker data to inform development strategy
-
Clinical trial management technology and EDC systems practices and prevalence
-
Outsourcing strategies and practices -
Personnel workload and capacity by R&D function -
FDA expedited development & review programs -
Orphan drug product protocol designs and development speed -
Impact of ICH E6 (R2) and E8 on functional operations and R&D operational risk
The following multi-company working groups are currently active:
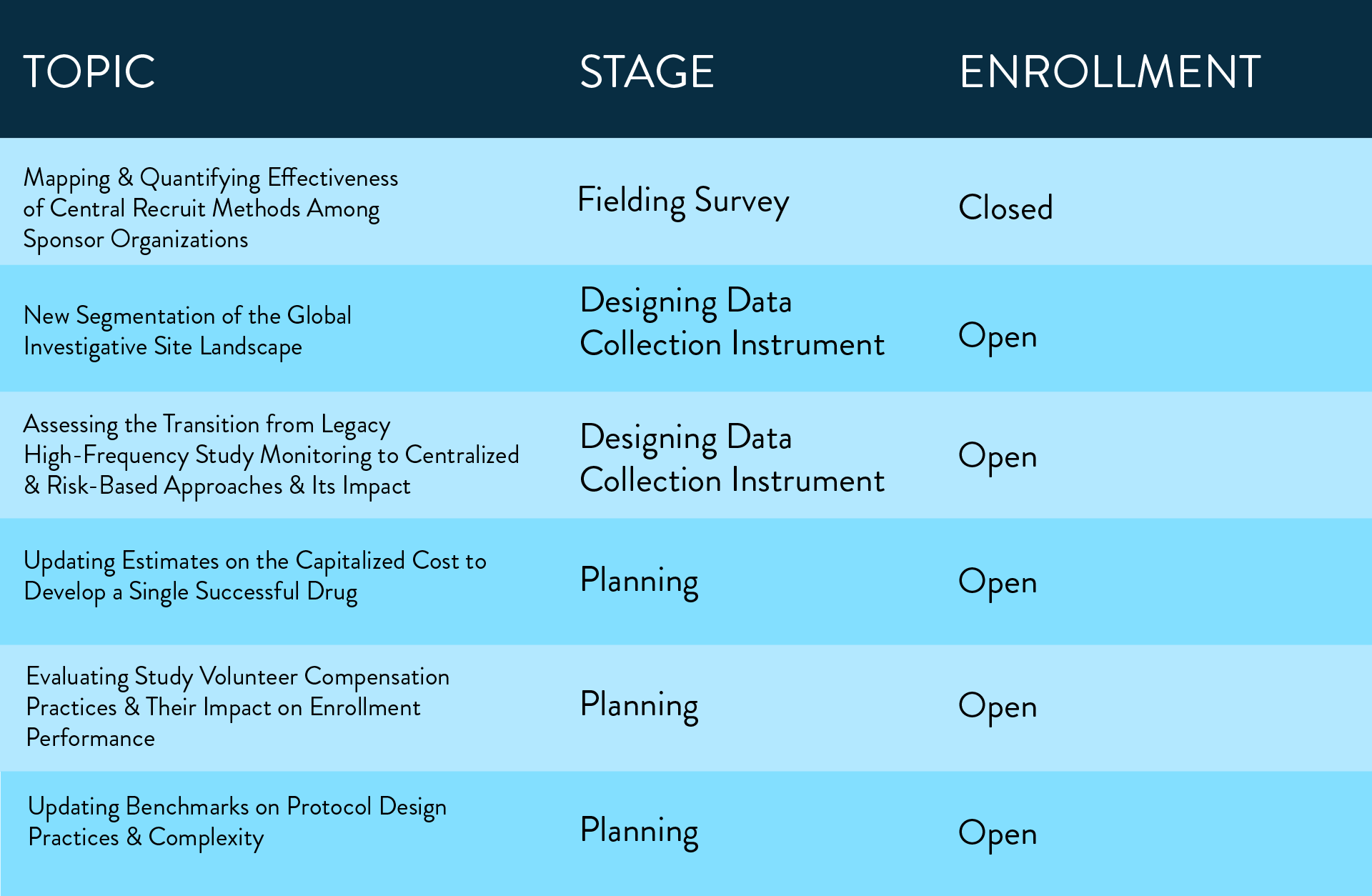
To discuss a research topic of interest and for more information about individual studies and multi-company working group studies: