Tufts CSDD produces authoritative White Papers to highlight current thinking on critical drug development issues. White Papers present findings and insights from Tufts Center analyses, as well as detailed reviews of roundtable discussions and forums hosted by CSDD. 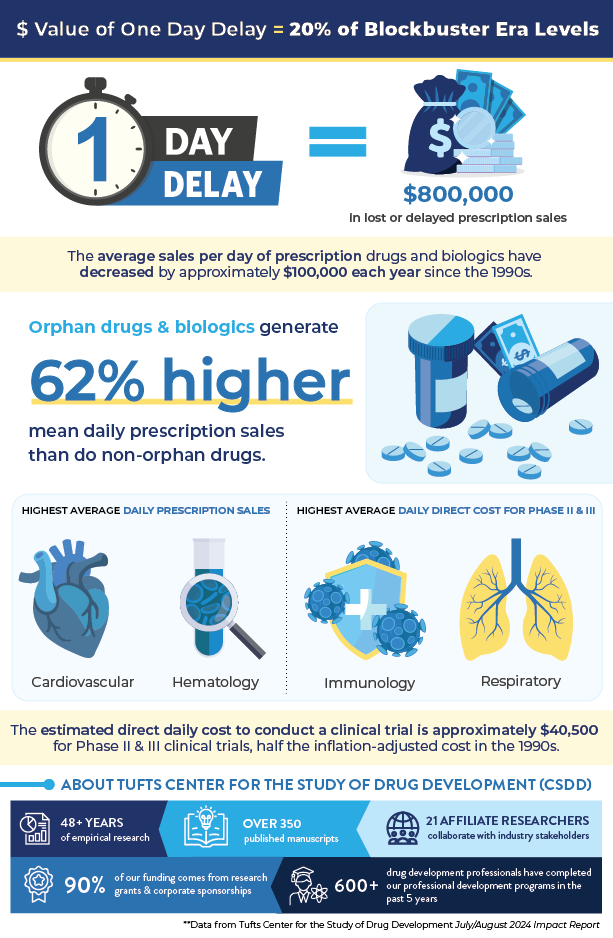
Quantifying the Value of a Day of Delay in Drug Development Infographic
(Summer 2024)

Quantifying the Value of a Day of Delay in Drug Development
(Summer 2024)

Comprehensive Summary of Site Engagement Literature
(Spring 2024)
Much has been written in the literature — both peer- reviewed and trade journals — about the unprecedented operating challenges that the global community of investigative sites face. This reference resource provides a comprehensive summary with citations and links to these articles, organized by challenge category. In all, this resource offers valuable insights informing site engagement strategies and practices.

Assessing Sponsor, Site, and Patient Receptivity to Retail Pharmacy Involvement in Clinical Trials
(Spring 2024)

Benchmarking and Optimizing the Process for Adopting Innovations Supporting Clinical Trial Execution
(Fall 2022)
Although there are conceptual frameworks and an extensive body of work in the literature examining the barriers and challenges associated with the adoption of new innovations supporting clinical trial execution, there is no empirical data benchmarking the process. As part of a working group study, Tufts CSDD conducted 26 in-depth interviews followed by an online survey that yielded 631 responses from the global community of drug development professionals. This study found that the four stages of the innovation adoption process – Initiation, Evaluation, Adoption Decision, and Full Implementation — takes 5.8 years on average with mid-sized companies taking one year longer than large companies and nearly two years longer than small companies. CROs are able to complete the innovation adoption process in half the time. High variation around the mean duration was observed overall and by company size showing highly inconsistent experience and the difficulty that companies face in navigating the process. The latter two stages of the process — Adoption Decision and Full Implementation — take the longest, are the most variable, and are regarded as the most difficult. Factors and approaches to accelerate the process and optimize innovation adoption are discussed.
%20Guidance.png)
Summary of themes from the
2021 Executive Roundtable Examining
Experiences Implementing and
Accommodating the ICH E6 (R2) Guidance
(JUNE 2021)
On May 2021, the Tufts Center for the Study of Drug Development — in collaboration with, and with funding from, CluePoints and PwC — hosted a virtual roundtable comprised of R&D senior executives from pharmaceutical and biotechnology companies, contract research organizations (CROs) and a representative from the Food and Drug Administration. In total, 60 people participated in a virtual, facilitated discussion.
Access a copy of this White Paper Report
Drug Development Workforce in the Age of Digital Transformation
(JUNE 2020)
On December 4, 2019, the Tufts Center for the Study of Drug Development (Tufts CSDD), with support by a grant from Pfizer, Inc., facilitated a first-of-its-kind Senior Leadership Roundtable in New York City to discuss the current state of the drug development workforce as it enters the digital era. Sixty executives from pharmaceutical and biotechnology companies, technology-solutions companies, professional trade organizations, and academia participated in the roundtable. Participant functional expertise included clinical, clinical operations, data management, regulatory affairs, human resources, digital technology, and R&D strategy. Experts discussed the current state and preparedness of the drug development workforce, challenges and opportunities presented by the digital transformation era, new and emerging critical skills and competencies required, changes in talent recruitment and retention practices, and the reshaping of corporate mindsets and cultures to become digitally proficient organizations. This White Paper, which provides key findings from the roundtable, is intended to inform and stimulate discussion as the various components of the drug development pipeline lead the vanguard in the Fourth Industrial Revolution. Access a copy of this White Paper Report
Assessing the Financial Impact of Translational Pharmaceutics - A Platform for Accelerating Product Development
(OCTOBER 2019)
Pharmaceutical R&D activity continues to grow significantly year-on-year with increasing numbers of molecules in development. Despite increases in spending, the industry struggles with poor R&D productivity, citing lengthy drug development times, increasing costs, and high rates of molecule attrition. Tufts CSDD examined an innovative approach to accelerating drug development, Translational Pharmaceutics®, and quantified the savings to drug developers from applying the approach across the industry portfolio of investigational drugs. Translational Pharmaceutics integrates real-time manufacturing and clinical testing to make drug products available for clinical trials more quickly and flexibly than is the case for traditional drug development. Translational Pharmaceutics projects were compared to industry benchmarks, and the financial benefits were quantified on reduced industry R&D costs and increased returns from earlier sales. Data were obtained for different types of Translational Pharmaceutics projects and topline results included mean total benefits ranging from $102.6 million to $290.1 million and mean timeline savings of >12 months. Access a copy of this White Paper Report.
ALPHA Project Unites Global Lupus Community on Barriers to Research, Drug Development, Care, and Access
(AUGUST 2019)
The study, “Global Consensus Building and Prioritization of Fundamental Lupus Challenges: The ALPHA Project,” brought together experts across 20 countries to provide the first-ever global consensus on key issues in lupus, which until they are addressed, will continue to be major barriers in the field. The ALPHA Project has identified fundamental knowledge gaps preventing advancements in lupus research, clinical care, and access and provides a framework to establish a roadmap for academia, federal agencies, industry, and all other lupus stakeholders to follow moving forward. The next phase of the ALPHA Project will be to organize an international stakeholder meeting with the Global Advisory Committee and other lupus stakeholders to be held in Washington, D.C. in January 2020, to implement specific solutions to address each barrier identified through this research. Access a copy of this White Paper Report..png)
Summary Report From the Spring 2019 Executive Roundtable on Company Response to ICH E6 (R2)
(JULY 2019)
On April 3, 2019, the Tufts Center for the Study of Drug Development held a second executive roundtable at the PwC offices in Boston, MA. The roundtable invited senior R&D leaders from biopharmaceutical companies to provide real-case sharing and discuss insights on the ICH E6 (R2) regulatory guidelines. The executive roundtable was produced and facilitated by Ken Getz, MBA, Research Associate Professor and Yaritza Peña, Research Analyst. Access a copy of this White Paper Report
Manufacturing Strategy for Diverse Biologic Pipelines of the Future
(OCTOBER 2017)
In October 2015, the Tufts Center for the Study of Drug Development hosted a workshop to explore innovative strategies for biologics manufacturing. The workshop was moderated by Abdullah Baaj, MD, PharmD, Parrish Galliher, MS, and Kenneth I Kaitin, PhD. This Tufts CSDD White Paper reviews the salient issues discussed and conclusions reached at the workshop, and explores the current state and future of biologics manufacturing.Access a copy of this White Paper Report
Assessing the Economics of Single-Source vs. Multi-Vendor Manufacturing
(OCTOBER 2017)
This report examines the impact on development costs and net returns for drug developers if clinical development cycle times are reduced, which has implications for development efficiency, innovation incentives, and earlier access by patients to new therapies. The analysis is conducted in the context of estimated differences in development times between single-source and multi-source outsourced contract manufacturing. The full study has been submitted for peer-reviewed publicationAccess a copy of this White Paper Report
Profiles of New Approaches to Improving the Efficiency and Performance of Pharmaceutical Drug Development
(MAY 2015)
This report details six primary areas across scientific, operating, and manufacturing divisions including new approaches to validating drug targets; integrating real world data into the R&D process; flexible and adaptive clinical trials; and green manufacturing techniques driving efficiency while reducing carbon footprint.Access a copy of this White Paper Report
Public and Private Sector Contributions to the Research & Development of the Most Transformational Drugs of the Last 25 Years
(JANUARY 2015)
In the current study (January 2015), Tufts CSDD authors examine a diverse array of evidentiary materials for 19 individual drugs, six drug classes and one drug combination, identified as the most transformative drugs in health care over the last 25 years, to better understand the respective contributions of the public and private sectors. The results of the analysis show that drug discovery and development is a complex ecosystem with a wide range of novel collaboration archetypes, involving industry-academic partnerships, venture capital, disease foundations, as well as public-private, pre-competitive consortia, so that learning is from many disciplines and the result of multiple feedback loops.Access a copy of this White Paper Report
Industry Usage of Social and Digital Media Communities in Clinical Research
(JUNE 2014)
Tufts CSDD convened a working group of 20 pharmaceutical and biotechnology companies, and contract research organizations (CROs) to assess current and anticipated use of social and digital media communities in clinical research; to identify challenges, receptivity, and concerns about usage; and to develop a comprehensive set of management principles and policies designed to optimize the value and minimize the risk posed by social and digital media use in clinical research.Access a copy of this White Paper Report
A New Tool for Predicting Marketing Approval of Oncology Drugs
(DECEMBER 2013)
Tufts CSDD and Janssen Research & Development (JRD) collaborated on a pilot study to develop and test a model capable of predicting the likelihood of marketing approval for oncology NMEs and NBEs. The study assessed the predictive power of select new compound characteristics. Access a copy of this White Paper Report
The Adoption and Impact of Adaptive Trial Designs
(MAY 2013)
Tufts CSDD hosted and facilitated a Senior Leadership roundtable on February 13, 2013 in Boston to discuss the adoption and impact of adaptive design clinical trials. Forty senior executives from a variety of cross-functional areas participated including clinical research and development, biostatistics, project management, and clinical operations. In addition, perspectives from the Food and Drug Administration and European Medicines Agency were represented.Access a copy of this White Paper Report
Academic-Industry Partnerships for Biopharmaceutical Research & Development: Advancing Medical Science in the U.S.
(APRIL 2012)
A new study by the Tufts University Center for the Study of Drug Development explores the breadth and nature of partnerships between biopharmaceutical companies and academic medical centers (AMCs).Access a copy of this White Paper Report